|
Back to Annual Meeting Posters
Novel Technique for the Creation of Patient-Specific Abdominal Aortic Aneurysms Phantom Models for the Study of Aortic Wall Failure
Doran Mix, B.S.1, Mallory Wingate2, Karl Q. Schwarz, M.D.1, Dan B. Phillips, Ph.D.2, Steven W. Day, Ph.D.2, Ankur Chandra, M.D.1.
1University of Rochester, Rochester, NY, USA, 2Rochester Institute of Technology, Rochester, NY, USA.
OBJECTIVES: Current size-based decisions for AAA repair can misrepresent individual risk of rupture. A specific patient’s rupture risk is related to tissue mechanics relative to wall stress created by AAA geometry and hemodynamics. Our hypothesis is that patient-specific AAA failure can be modeled in vitro using a novel molding technique using tissue mimicking material, Polyvinyl Alcohol Cryogel (PVA-C), and physiologic pulsatile hemodynamic stress.
METHODS: 3-dimensionial stereolithography (STL) CAD models are rendered from patient CT-angiograms using Mimics® image processing software (Figure 1A). Hoop stress is calculated in Matlab® from the model geometry during peak systolic loading with a heat map to show points of relative maximum stress (Figure 1B). The model is printed as solid plastic form, using a Dimension® 3D printer (Figure 1C). Separate silicon casts are created of both the inner and outer vessel wall lumens. A low temperature wax model is created from the inner lumen cast and assembled into the outer lumen cast to create the final mold. The elastance of the PVA-C material is programmed through a sequence of cyclic freeze-thaw cycles. The model is mounted onto an In vitro hemodynamic simulator and tested with physiologic pressure and flow until failure (Figure 1D). A combination of high-speed video (500 frames/s), 2D ultrasound, and UV-fluorescence intensity imaging are used to determine the exact location of failure and wall thickness and aneurysmal radii at that time.
RESULTS: PVA-C models dimensions of radii and wall thickness accurately mimicked patient geometries as determined by 2D ultrasound with +/- 10% tolerance. UV-fluorescence intensity imaging corresponded to relative vessel wall thickness when compared with STL CAD model wall thickness. Hoop stress heat maps accurately predicted the location of PVA-C model failure, during pulsatile physiologic stress, as determined by high-speed video analysis.
CONCLUSIONS: We have created a novel technique for the creation of a low cost in vitro model of patient specific aneurysmal geometries. This work will form the basis for testing new modalities of rupture risk assessment; including the use of transcutaneous ultrasound stain analysis for the determination of patient specific rupture risk to determine the need for operative intervention. 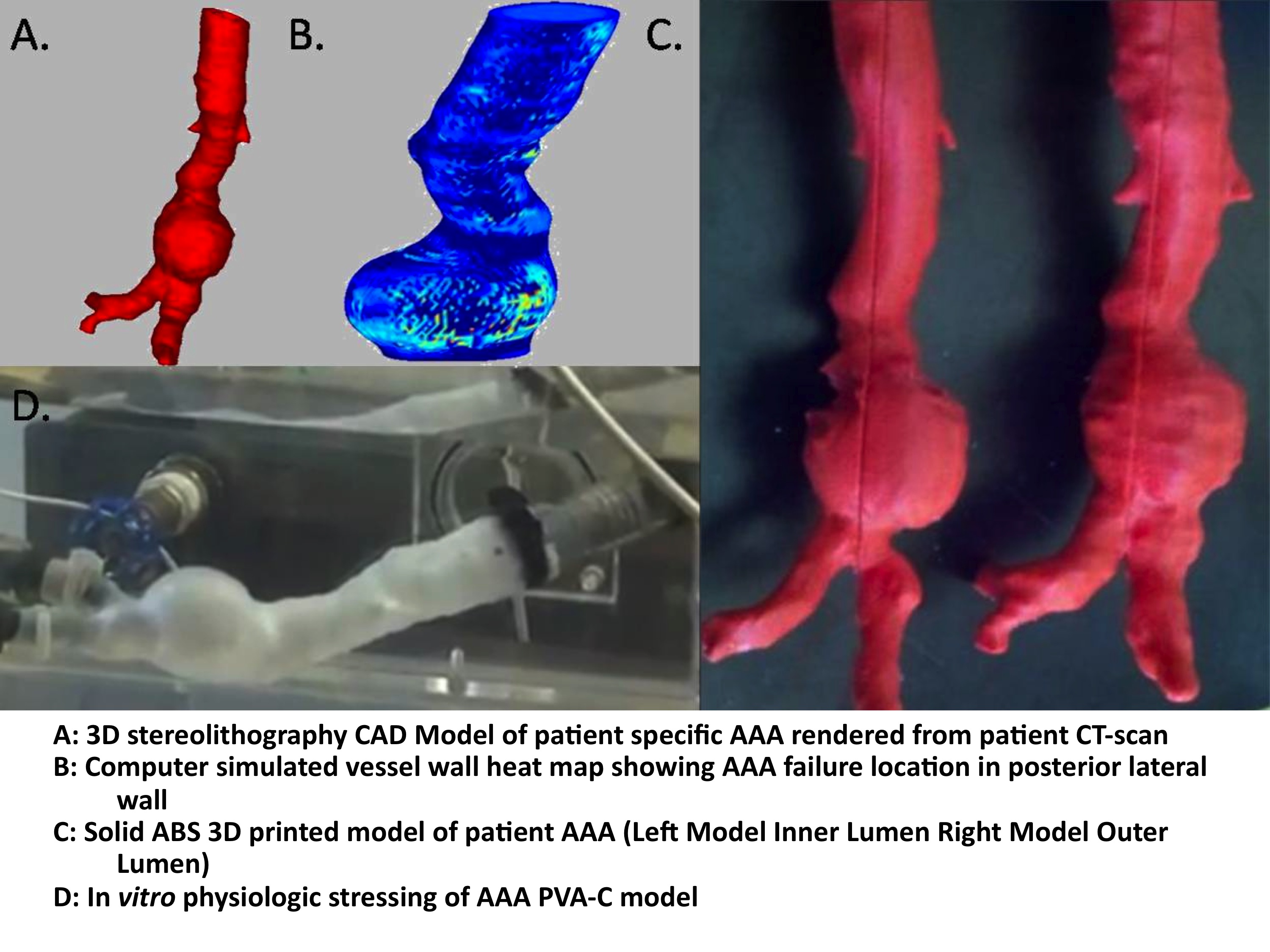
Back to Annual Meeting Posters

|


