One-year Results of the (ROADSTER) Multicenter Trial of Transcarotid Stenting with Dynamic Flow Reversal
Mahmoud B. Malas, MD1, Besma J. Nejim, MBChB MPH1, Jose Ignacio Leal Lorenzo, MD2, Manish Mehta, MD MPH3, Todd M. Hanover, MD4, Vikram S. Kashyap, MD5, Richard Cambria, MD6, Christopher J. Kwolek, MD6.
1Johns Hopkins University School of Medicine, Baltimore, MD, USA, 2Complejo Hospitalario de Toledo, Toledo, Spain, 35Albany Vascular Group, The Institute for Vascular Health and Disease, Albany, NY, USA, 4Greenville Hospital System, Greenville, SC, USA, 5University Hospitals Case Medical Center, Cleveland, OH, USA, 6Masssachusetts General Hospital, Boston, MA, USA.
Objective: The aim of this study is to report the one-year outcomes of the Transcarotid artery stenting (TCAR) with cerebral blood flow reversal (ROADSTER) multicenter trial outcomes and to evaluate the durability of TCAR. Overall 30-day stroke rate was 1.4% in the ROADSTER trial which was the lowest reported in any randomized clinical trial for carotid artery stenting.
Methods: This study is a prospective, single-arm clinical trial. Primary end points were incidence rates of ipsilateral stroke and death at one-year following (TCAR). Occurrence of stroke was ascertained by an independent clinical event committee.
Results: 219 patients were enrolled (pivotal phase: 141, extended access: 78 patients). Of those, 164 patients were included in the one-year follow-up (16 had postoperative events, 3 died, 10 declined to participate, and 26 were lost to follow-up). Mean age was 73.9 years (range: 42.1-91.3 years). 43.3% of patients were at least 75 years old. 34.8% of patients were females, 92.7% were Caucasians and 5.5% were African-American. Most patients were asymptomatic (79.9%). Patients with anatomical risk factors were distributed as follows: contralateral carotid artery occlusion (11.0%), tandem stenosis of <70% (1.8%), high cervical carotid artery stenosis (25.0%), restenosis after CEA (25.6%), bilateral stenosis requiring treatment (4.3%) and hostile neck (14.6%) of cases. Physiological high-risk factors included two-vessel coronary artery disease (14.0%) and severe left ventricular dysfunction (LEVF<30%) was reported in 3 (1.8%) patients. At one-year follow-up, ipsilateral stroke incidence rate was 0.6% and overall mortality rate was 3.7%. The one-year risk of stroke found in this study is the lowest to date to be reported in any FDA-approved carotid stents (Figure).
Conclusions: TCAR with dynamic flow reversal had previously shown favorable 30-day perioperative outcomes. This excellent performance seems to extend to one year following TCAR as illustrated in this analysis. The promising results from the ROADSTER trial might stem from the novel cerebral protection provided through the ENROUTE Transcarotid NPS in comparison to distal protection devices. The trans-cervical approach circumvents aortic arch manipulation that takes place through the trans-femoral approach. TCAR offers a potentially safe alternative option for patients who are deemed to be high-risk for CEA. 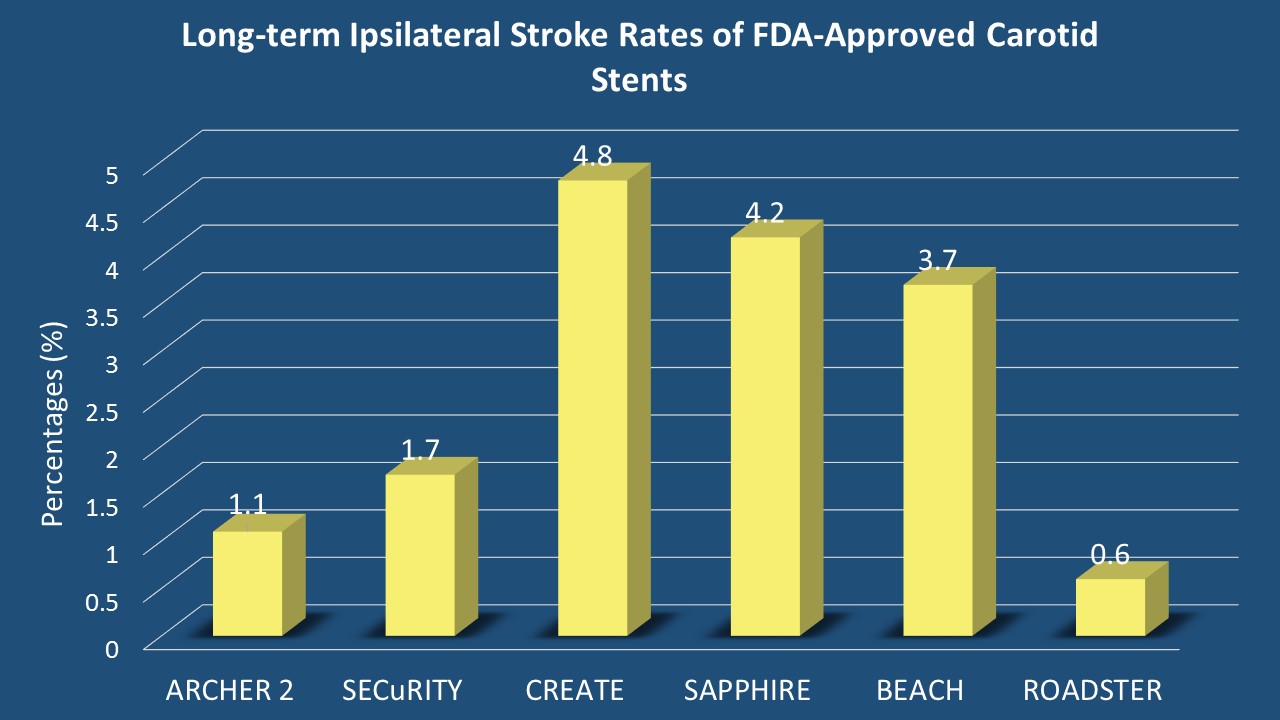
Back to 2018 ePosters




