Peripheral aneurysm coiling using large volume Ruby coils: Results from the multicenter ACE trial
Frank R. Arko, III, MD.1, Parag Patel, MD.2, Blaise Baxter, MD3, J. David Moskovitz, MD4, Henry Moyle, MD.5, Ryan Hagino, MD.6, Richard Klucznik, MD.7, Don Heck, MD.8, Philippe Gailloud, MD.9.
1Sanger Heart and Vascular institute, Carolinas Health Care System, Charlotte, NC, USA, 2Medical College of Wisconsin, Milwaukee, WI, USA, 3Erlanger Health System, Chattanooga, TN, USA, 4Florida Hospital,, Orlando, FL, USA, 5Mount Sinai Medical Center, New York, NY, USA, 6Essentia Health, Duluth, MN, USA, 7The Methodist Hospital Research Institute, Houston, TX, USA, 8Forsyth Medical Center, Winston-Salem, NC, USA, 9Johns Hopkins University, Baltimore, MD, USA.
Objective
ACE trial aims to demonstrate safety and efficacy of soft, large volume, bare platinum RubyTM Coils in achieving high packing density, leading to complete aneurysm obliteration and stable vessel embolization.
Method
The Aneurysm Coiling Efficiency (ACE) trial was a prospective, multicenter study, designed to capture data on safety and efficacy of large volume coils in peripheral vascular embolizations. Patients were identified from 15 centers between March 2012-December 2016. Patients within the ACE registry were categorized into aneurysm embolization or vessel sacrifice subgroups. Primary outcomes include packing density fluoroscopic exposure, procedural device-related serious adverse events (SAE), and occlusion status at 6-months with an optional 1-year outcome.
ResultsA total of 78 cases were treated in 67 patients. Median cohort age was 59 years [IQR 48-71]; 44.8% were female. In cases involving aneurysms (n = 26), a median of 6.5 coils [IQR 4-14] were deployed, achieving a median packing density of 26.8% [IQR 18.6-33.0] per case. Median fluoroscopy time was 29.0 minutes [IQR 21-36]. At six month follow-up 90.9% (20/22) of cases achieved stable or better occlusion grade compared to post-procedure and 100.0% (15/15) at one year follow-up.
Successful embolization was achieved in all vessel sacrifice or miscellaneous malformations. A median of 3 coils [IQR 2-5] were placed in a median of 21.5 minutes [IQR 15 - 29.0] under fluoroscopy. In cases with 6-months, 91.7 % (22/24) , and 1-year 100.0% (3/3) follow-up, demonstrated stable or better occlusion grade than post-procedure.
Common sites for embolization of this cohort include pulmonary (n=5), splenic (n=12) and gastroduodenal artery (GDA, n=10). Compared to GDA patients in a Maleux’s study1 wherein fibered and hydrogel coated coil technology were employed, significantly fewer Ruby Coils were required to embolized the GDA.
Only, 4.5% (3/67) of patients reported SAEs within 24-hours post-procedure, none were device related.
Conclusion
RubyTM coil efficiently achieves high packing density and stable occlusion in aneurysm embolization and vessel sacrifice. Six month and 1-year follow up support long-term embolization stability in both cohorts.
Footnotes
1Journal of Vascular and Interventional Radiology. 2013 Jun 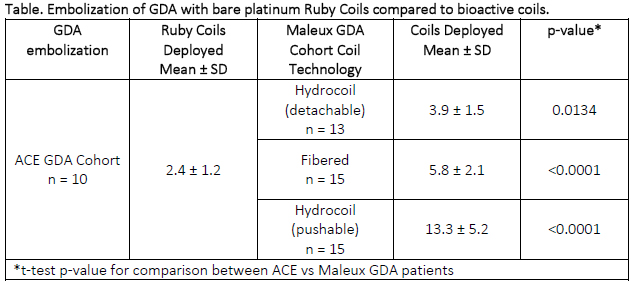
Back to 2018 ePosters




