IS INCIDENCE OF GRAFT THROMBUS AFTER TEVAR FOR THORACIC AORTIC INJURY HIGHER IN CERTAIN DEVICES?
Sashi K. Inkollu, M.B.,B.S., Samantha Minc, MD, Eric Shang, MD, Luke Marone, MD.
West Virginia University, Morgantown, WV, USA.
Objective:
There has been a recent recall of alpha thoracic devices (18-22 mm) and removal of blunt thoracic aortic injury (BTAI) as an indication for its use due to reports of graft thrombus. Current long term follow up data on TEVAR performed for aortic trauma is limited. Risk of thrombus formation may not be unique to the Cook device
Methods:
We performed a chart review of TEVAR performed for BTAI in our center during 2008 to 2017 and examined available postoperative CT scans. Patients without follow up within 1 year were contacted in an attempt to obtain imaging
Results:
23 patients underwent TEVAR for traumatic thoracic aortic injury at our institute during 2008 to 2017. 22 were for BTAI and 1 for penetrating aortic injury. There were 5 deaths in the immediate postoperative period. Among the 18 survivors, devices used included TAG (5), CTAG (4), Valiant (4), Talent (3), GORE iliac limb(1) and Alpha (2). Median follow up was 3.5 months. 7 patients were treated with device sizes between 18-22mm (Alpha 2, Talent 3, Valiant 1, CTAG 1 and GORE iliac limb 1). The last follow-up scan was available at 10 days, 2 months, 3 months, 7 months, 25 months, 56 months and 76 months’ duration postop for these patients. One of them had a persistent postoperative type 1a endoleak after a 20mm GORE iliac limb, requiring a proximal extension with a 22mm TALENT. The same patient also developed graft thrombus within both the TALENT and GORE components which was first noticed at 6 months and progressed on CT imaging at 54 months. Graft thrombus has been stable since with <50% luminal narrowing
Conclusions:
In our retrospective review of TEVAR for traumatic thoracic aortic injury, luminal thrombus was noted in small devices other than alpha. No patients with small alpha devices or all other larger devices were noted to develop graft thrombus although follow up was quite poor. Further study with larger samples is needed to assess safety of small devices and better follow up strategies will be instituted at our institution and should be considered on a national scale 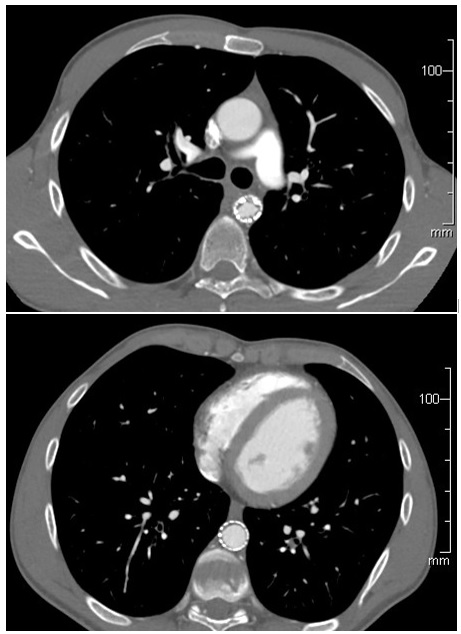
Back to 2018 ePosters




