Multi-Institutional Implementation of a Vendor Rebate Model to Reduce Costs
Edward J. Arous, M.D.1, Calvin L. Chao, B.A.1, Houman S. Tamaddon, M.D.2, Jessica P. Simons, M.D., M.P.H.1, Danielle R. Doucet, M.D.1, Dejah R. Judelson, M.D.1, Andres Schanzer, M.D.1, Francesco A. Aiello, M.D.1.
1University of Massachusetts Medical School, Worcester, MA, USA, 2University Vascular Specialists, Augusta, GA, USA.
Objective
Novel and costly endovascular devices play an increasingly common role in the treatment of lower extremity peripheral artery disease. In an evolving health care environment, identifying cost-containment measures has become critically important. The Medicare Access and CHIP Reauthorization Act (MACRA) will employ patient care costs in determining professional reimbursement through Merit-based Incentive Payment System (MIPS) and Alternative Payment Model (APM). We provide a proof-of-concept model utilizing next-generation devices through a vendor rebate system for controlled cost reduction. We hypothesize that future extrapolation to multiple vendors may allow for a risk sharing cost-containment model.
Methods
We conducted a multi-institutional retrospective review of superficial femoral artery (SFA) interventions, utilizing a single vendor rebate system for drug-eluting stents, at two US institutions (9/2016-7/2017). The rebate included an all-inclusive single vendor cost model, whereby vendor products were part of a fixed cost rebate system. Inclusion criteria included SFA primary intervention with or without secondary intervention in another lower extremity vessel. Vendor case savings were calculated by deducting the rebate amount from total vendor costs. Total case savings were calculated by deducting the rebate savings from total case cost, irrespective of intervention or product utilization.
Results
The initial 20 cases at site 1 utilizing the rebate consisted of isolated SFA interventions (65%), SFA interventions with a secondary intervention (30%), and SFA intervention with two secondary interventions (5%). On average, 1.7 stents were used per case. At site 1, the rebate system resulted in a 21% mean vendor cost savings and a 14% mean total case savings ($650.44/case). Using the identical rebate model at site 2, 37 cases utilized the rebate system resulting in a 28% mean vendor cost savings ($1443.76/case) (Figure).
Conclusions
A single vendor rebate model resulted in a savings in vendor costs by 21% and 28% at two institutions. Total case savings were 14% after the rebate. As MACRA proposes linking physician reimbursement to quality and cost containment, physicians will need to play an integral role in controlling patient care costs. This study demonstrated that costs savings can be secured through a rebate system that distributes risks between physician, institution, and industry. 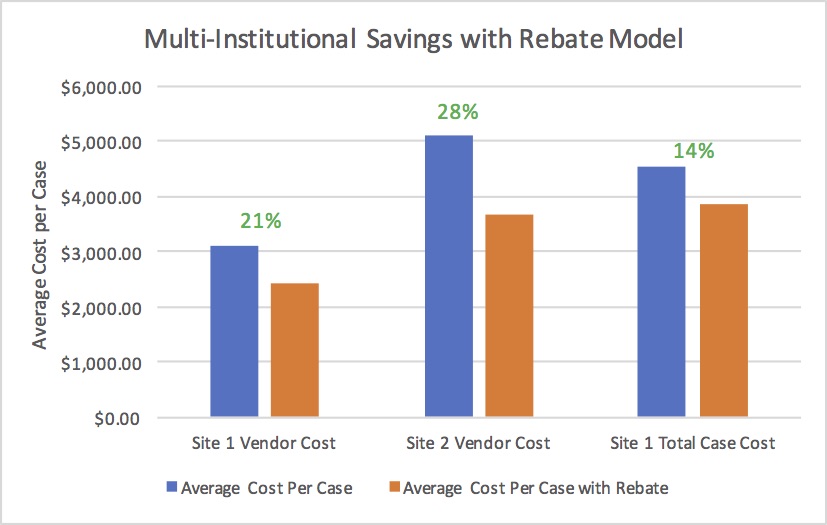
Back to 2018 ePosters




