Chasing Red Flags: A Maude Analysis Reveals Endoleak Signals In A Recalled Evar System
James Dittman, Michael F. Amendola, MD.
VA Medical Center/VCU Health System, Richmond, VA, USA.
BACKGROUND: The Manufacturer and User Facility Device Experience (MAUDE) Database was established for voluntary reporting of adverse outcomes with medical devices. The practical utility of this database has been criticized because lack of context, underreporting, and duplicate reporting limits the interpretation of aggregate reports. The AFX EVAR System (AFX; Endologix Inc) was recalled with warning from the FDA on July 20, 2018 due to reports of Type III Endoleaks (T3E). We set forth to examine the MAUDE for both systems to determine the rate of T3E and other adverse events reported.
METHODS: The MAUDE database was accessed in at a single time point on December 1, 2018 and all events reported from October 12, 2015 to October 31, 2018 related to key word search of “AFX” or “AFX2” linked to the Endologix company were compiled. Event descriptions were analyzed to remove reports pertaining to AFX implantation prior to October 12, 2015 to limit sampling to the same time period. Average issues per report, mortality reported, overall leak, failure to seal, migration mentioned, and device occlusion were collected. T3E was defined as reports of tear or component separation were also collected. Fisher’s exact* and two tailed t-test** were utilized to test for significance.
RESULTS: A total of 1,182 entries were examined, of which 990 (or 83.8%) were entries of adverse reports for AFX and 192 (or 16.2%) were entries of adverse reports for AFX2. There were more average issues per report for AFX vs. AFX2 (2.4 ± 1.2 vs. 2.0 ± 1.1;p=0.0001**) respectively. There were statistically significantly reported mortality (10.9% vs. 3.6%;p=0.0001*) and device occlusions (14.6 vs. 4.3%;p=0.0001*) for AFX2 compared to AFX respectively.
CONCLUSIONS: The prevalence of T3E in adverse reports was significantly higher with the AFX system than AFX2, supporting the national AFX recall without recall of AFX2. Interestingly, the newer generation AFX2 grafts showed higher reported mortality and occlusion rates in MAUDE. This data should encourage the vascular community that MAUDE, despite its known limitations, can indicate potential device difficulties before such devices would otherwise be recalled by manufacturers. 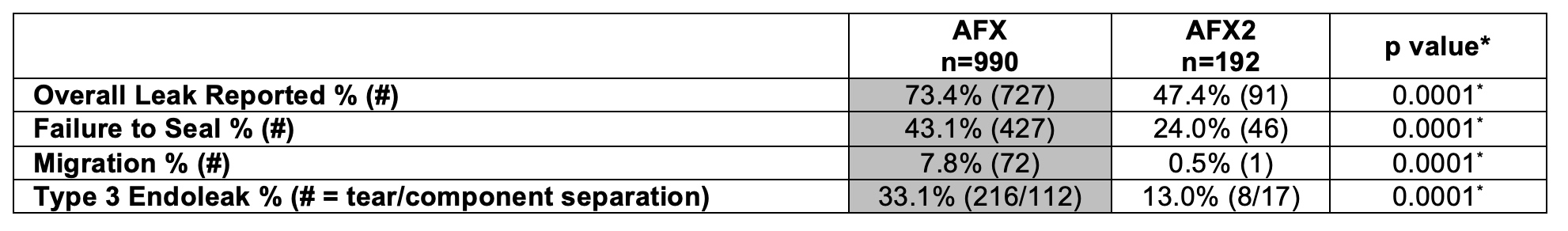
Back to 2020 ePosters
