What Is The Signal? A Ten-year Mortality Report Analysis Of The Maude Database For Paclitaxel Coated Peripheral Balloons And Stents
Michael F. Amendola, MD, James Dittman.
VA Medical Center/VCU Health System, Richmond, VA, USA.
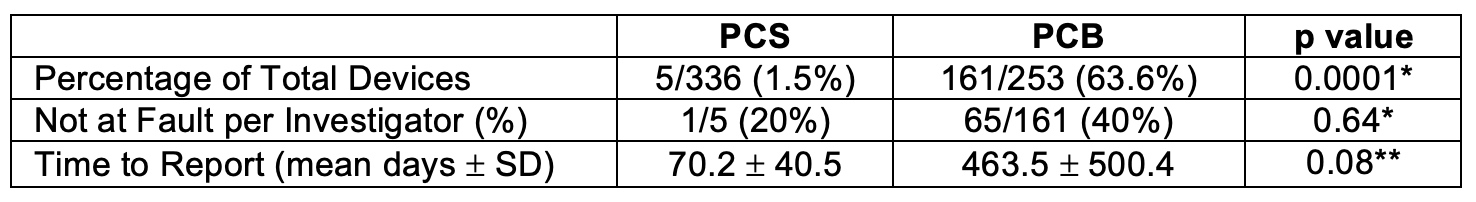
OBJECTIVES: Recently published meta-analysis has suggested an increased mortality signal at 2 and 5 years post-implant for Paclitaxel coated balloons (PCB) and/or stents (PCS). We set forth to examine all mortalities voluntarily reported to the FDA in the Manufacturer and User Facility Device Experience (MAUDE) Database over a period of ten years to compare deaths associated with Paclitaxel coated devices to uncoated devices and to the proportion of death reports attributed to vascular devices as a whole.METHODS: All adverse events death reports filed between April 1, 2009 to March 31, 2019 were compiled from the MAUDE database. Reports were attributed to vascular use based on reported device brand and those regarding stents and dilation balloons were further screened for peripheral utilization. The presence of Paclitaxel coating, presence of investigator judgement stating death was not fault of device, and average days from death to report filing were compared between PCB and PCS using Fisher’s Exact Test* and t-test**.RESULTS: During the study period a total of 111,976 death reports relating to medical devices were filed in MAUDE. Of these 8,104 (7.2%) pertained to devices utilized in vascular surgery and 589 specifically to peripherally utilized balloons and/or stents were found. The majority of these (n=336) concerned stents of which only 5 or 1.5% were PCS. Of the 253 balloon related death reports in MAUDE, 161 (63.6%) were PCB. Comparison of investigator not at fault statements were not significant between PCS and PCB reports (20% vs. 40%; p=0.64*). No investigator notes in MAUDE reports implicated PCS or PCB.CONCLUSIONS: Over the last decade, few MAUDE death reports have been filed concerning PCS while PCB reports represent a majority of reports concerning peripherally utilized balloons. If a late mortality signal exists, it may differ between PCS and PCB. Less than half of PCS and PCB reports contained investigator notes and no investigation stated mortality was due to PCS or PCB usage, indicating discordance between the FDA’s large self-reported database as compared to ongoing industry based and FDA mandated study of Paclitaxel coated devices.
Back to 2020 ePosters
