Death By Device: A Ten-year Analysis Of Mortality In The Maude Database
James Dittman, Michael F. Amendola, MD.
VA Medical Center/VCU Health System, Richmond, VA, USA.
OBJECTIVE:The FDA has recently come under scrutiny for granting alternative summary reporting to thousands of medical devices, a policy which has resulted in over 1 million adverse event reports bypassing inclusion in the publicly searchable MAUDE database since 2016. Diminished transparency in post-market monitoring is especially concerning in vascular surgery where devices play a key role. Since deaths cannot be exempted from mandatory reporting, we set forth to survey every reported mortality in the MAUDE database over ten years to compare mortality trends for vascular devices against those across other surgical specialties using an estimate the percentage of reports addressed through device recalls.METHODS: All mortality events reported to MAUDE from April 1, 2009 to March 31, 2019 were compiled. 11,739 device models were categorized based on use and counts of brand model presence were used to sum reports in each category. Device models were classified as recall-linked via presence in FDA recalls of the International Consortium of Investigative Journalists database. Two-tailed Chi squared test with Yates correction** was applied.RESULTS: 111,976 mortality reports were filed in MAUDE over ten years, of which 111,232 indicated a specific device model. 66,755 (60.0%) concerned a surgical device. 5,201 vascular reports were associated with a recall-linked device brand. CONCLUSION: Vascular devices represent a small fraction of overall medical device recalls while ranking second only to cardiothoracic specific devices for highest rate of associated mortalities. The percentage of recall-linked reports in vascular surgery matches or exceeds that of all other surgical specialties, suggesting that effective post-market surveillance of vascular devices has been equal or superior to every other surgical specialty over the last decade. Determination of recall-linked mortality reports is longitudinally reproducible and could be utilized to indicate changes in the efficacy of post-market monitoring. 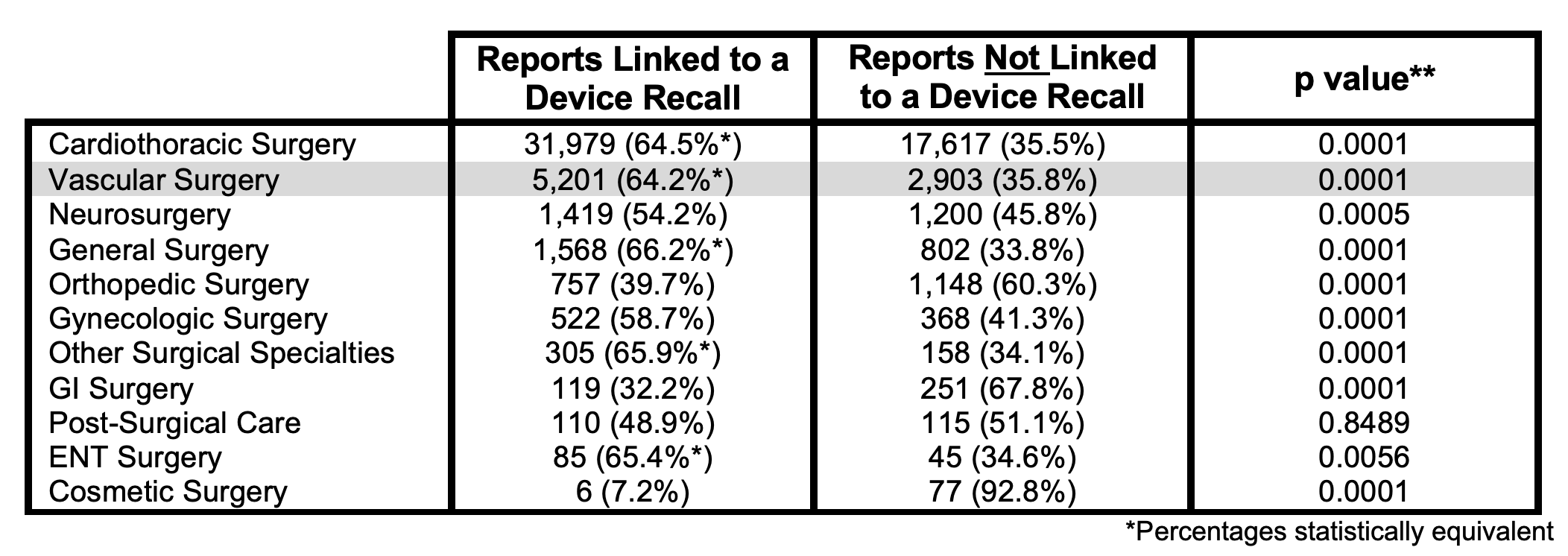
Back to 2020 ePosters
