Impact Of Combining Aortic Components From Multiple Manufacturers On Outcomes After Physician Modified Fenestrated, Branched Endovascular Aortic Aneurysm Repair
Alyssa J. Pyun, MD, Gregory A. Magee, MD, Kenneth Ziegler, MD, Louis Zhang, MD, Fred A. Weaver, MD, Raquel Caldera, PA, Sukgu M. Han, MD.
University of Southern California, Los Angeles, CA, USA.
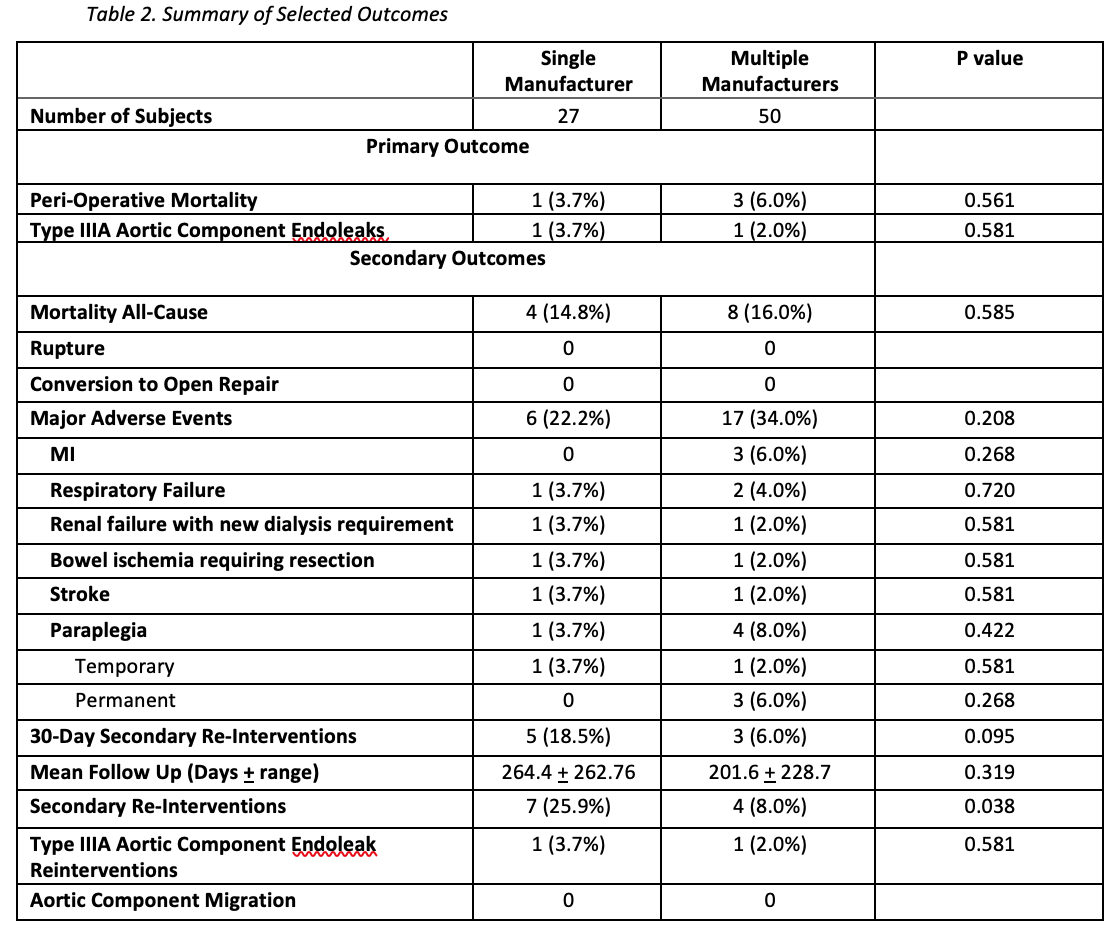
OBJECTIVE:Endovascular aortic repair technology continues to evolve by offering a variety of purpose-built devices.Combining components from different manufacturers, each with unique features for specific anatomymay be advantageous in complex endovascular aortic repairs. However, there is limited data evaluatingthe interaction between aortic components from different manufacturers, as well as the impact onclinical outcomes. Thus, our study aims to compare type III endoleak rates and clinical outcomesbetween patients who underwent physician-modified fenestrated, branched endovascular aortic repair(PM-FBEVAR) using aortic components from a single manufacturer, and those with aortic componentsfrom multiple manufacturers.METHODS:Retrospective review of consecutive patients who underwent PM-FBEVAR at a single institution fromSeptember, 2015 to October, 2019 was performed. Patient demographics, intraoperative details, andclinical outcomes were captured from electronic medical records. Anatomic details and endoleaks wereobtained from pre and post-operative CT scans. Type III endoleak between aortic components andperioperative mortality were the primary outcome variables. Fisherís exact test and Studentís t-testwere used to compare categorical and continuous variables. Data from this cohort were reported to theFDA, leading to approval of a physician (SH)-sponsored IDE (G200159) for physician modifiedendografting.RESULTS:During the study period, 117 patients underwent PM-FBEVAR at our institution. Patients withoutconcomitant infrarenal component placement were excluded resulting in 27 patients with PM-FBEVARcomposed of single manufacturer aortic components, and 50 patients with multiple manufacturer aorticcomponents. Patient demographics and comorbidities were not significantly different between the twogroups. There was no difference in type III endoleaks between aortic components and associatedreinterventions (3.7% vs 2.0% respectively, P=0.581), or in perioperative mortality (3.7% vs 6%respectively, P=0.561) between the single and multiple manufacturer group. There were noperioperative ruptures or conversion to open in either group. At mean follow-up of 223.6 days,secondary reinterventions, addressing branch complications and type Ia endoleak, were more frequentin the single manufacturer group compared to the multiple manufacturer group (25.9% vs 8%respectively, P=0.038).CONCLUSION:Combining aortic components from different manufacturers during PM-FBEVAR did not result inincreased perioperative mortality or type III endoleaks. Long term follow-up is planned to assessdurability. 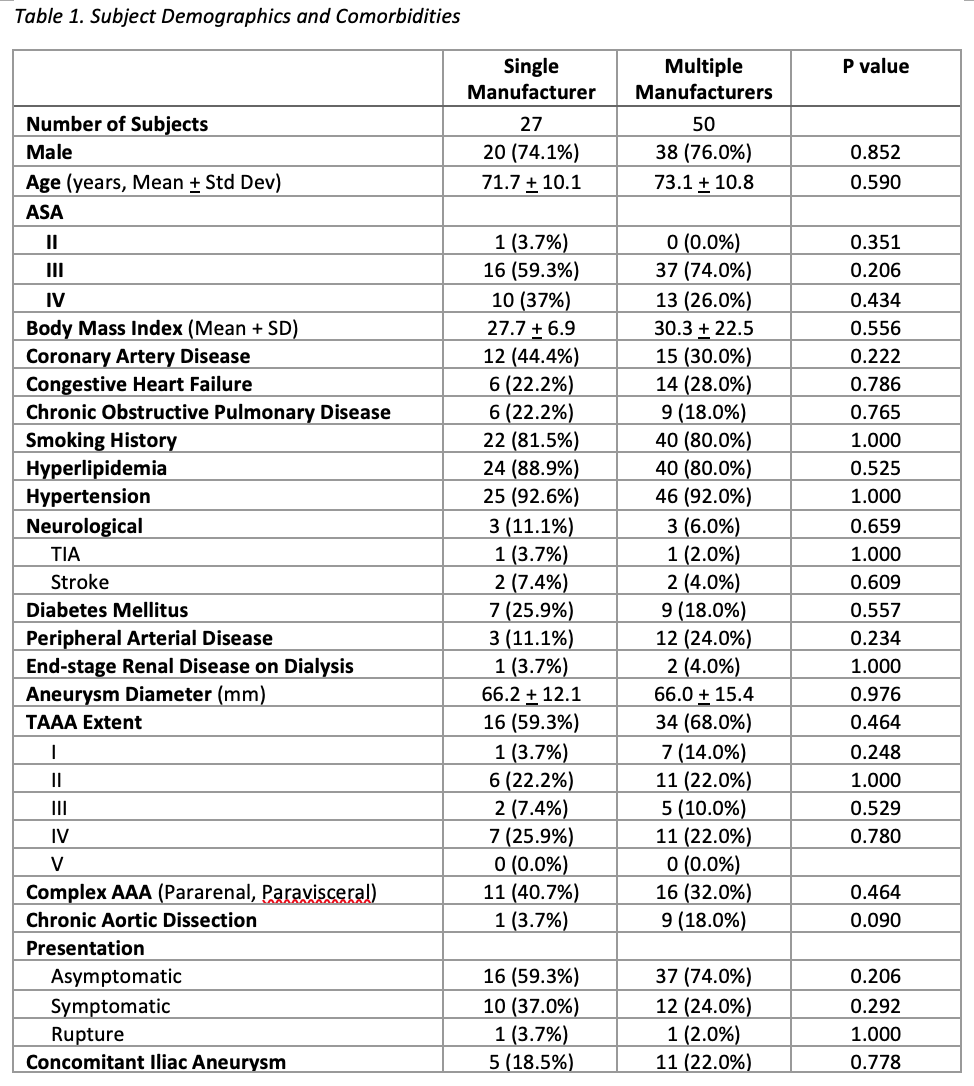
Back to 2021 Karmody Posters
