Initial Experience With The Flowtriever Mechanical Thrombectomy Device For Pulmonary Embolism
Hunter M. Ray, MD, Alejandro Pizano, MD, Naveed U. Saqib, Stuart A. Harlin, MD.
The University of Texas Health Science Center at Houston, Houston, TX, USA.
OBJECTIVES: The Inari FlowTriever (Inari Medical,Irvine,CA) is the first mechanical thrombectomy device indicated for pulmonary embolism approved. We review our initial experience with the INARI FlowTriever device (IFTD) for treatment of acute pulmonary embolism (PE). METHODS: All patients undergoing mechanical thrombectomy with the IFTD for PE were described and analyzed. Outcomes evaluated included technical success, major adverse events (MAE), thrombolytic drug administration, intensive care unit (ICU) admission and symptomatic improvement. Technical success is defined as successful thrombus removal with angiographic evidence of improvement.
RESULTS: During the study period, 11 patients with PE underwent mechanical thrombectomy with the IFTD. The cohort had a median (Interquartile Range; IQR) age of 60 (47-65) years, with 7/11 (63.6%) male, and the body mass index median was 34 (30-39) kg/m2. All the patients had right heart strain as the main indication for thrombectomy. Procedure median length was 45 (37-78) minutes, fluoroscopic time 10 (7-14) minutes, radiation 95.506 (83.642-194.528) cGycm2. There was 100% technical success, thrombolytic drug was used in one patient (9.1%), 3/11 (27.3%) required implantation of inferior vena cava filter, 5/11 (45.5%) patients were in the Intensive Care Unit (ICU), 3/5 required one day in the ICU, 2/5 required 5 days in the ICU, the rest of the patients who were not in the ICU did not needed ICU management after the procedure because of instability, and all patients (11/11[100%]) experienced improvement in symptoms at the time of discharge. No complications or MAEs. Preprocedural median hemoglobin was 13 (12-15) and pre-discharge was 12 (10-14), glomerular filtration rate was 78 (69-95), and pre-discharge was 89 (82-101). Overall post-procedural length of stay (post-LOS) was 3 (2-6) days. CONCLUSIONS: Our initial experience with the IFTD for treatment of PE is promising and appears a safe and effective with 100% technical success, no MAEs, low thrombolytic agents used, and short ICU utilization/post-LOS. Further studies are warranted, especially to evaluate this procedure against other approaches and long-term outcomes. 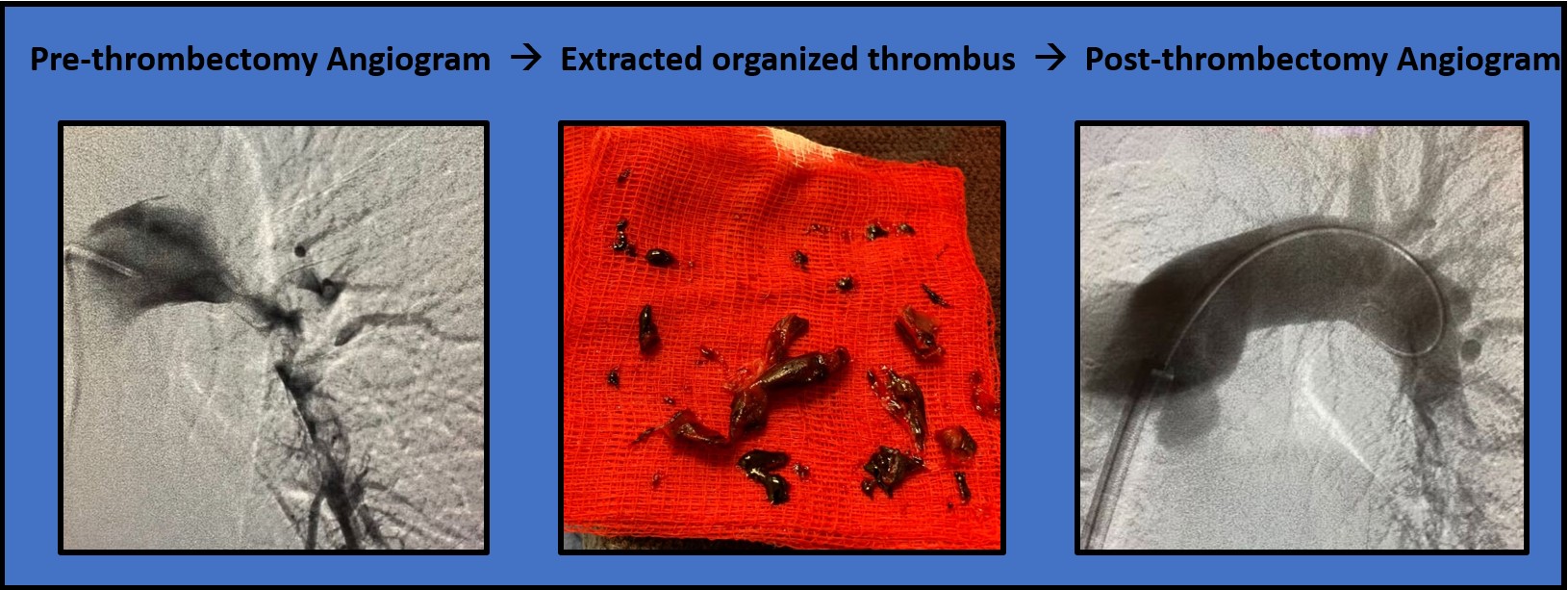
Back to 2021 Abstracts
