The Impact Of Operative Approach On In-hospital Outcomes After Carotid Stenting
Aderike C. Anjorin, MPH1, Christina L. Marcaccio, MD, MPH1, Priya B. Patel, MD, MPH1, Vinamr Rastogi, MD1, Douglas W. Jones, MD2, Mark C. Wyers, MD1, Marc L. Schermerhorn, MD1.
1Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA, 2UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA.
OBJECTIVE: Carotid artery stenting (CAS) is often used for patients at high risk for endarterectomy. Though performed with increasing frequency, there are limited data comparing transradial or transbrachial (tr/tbCAS) access to established CAS approaches. Therefore, our objective was to examine the effect of a tr/tbCAS approach versus a transfemoral (tfCAS) or transcarotid (TCAR) approach on outcomes after CAS.
METHODS: We identified all patients undergoing CAS in the Vascular Quality Initiative registry from January 2016 to March 2021. Our primary outcome was a composite endpoint of in-hospital stroke/death. Secondary endpoints included in-hospital stroke, death, myocardial infarction (MI), hyperperfusion symptoms, and bleeding complications. To assess these outcomes by CAS approach, we generated propensity scores incorporating demographic characteristics, symptom status, pre-operative medications, and comorbidities and then used inverse probability-weighted log binomial regression with adjustment for protamine use after model construction.
RESULTS: Among 36,328 CAS patients, 651 underwent tr/tbCAS, 17,731 underwent tfCAS, and 17,946 underwent TCAR (Table). In patients with symptomatic carotid disease, the rate of the composite endpoint of stroke/death was 4.5% with tr/tbCAS, 3.7% with tfCAS, and 2.1% with TCAR. In asymptomatic patients, the rate of stroke/death was 1.7% with tr/tbCAS, 0.9% with tfCAS, and 1.0% with TCAR. After adjustment, tr/tbCAS was associated with a significantly higher risk of stroke/death when compared with tfCAS [relative risk [RR]:1.5, 95%CI(1.0-2.3)] or TCAR [RR:1.8, 95%CI(1.2-2.9)]. Furthermore, tr/tbCAS was associated with higher risk of death [tr/tbCAS:2.2%, tfCAS:1.2%, TCAR:0.4%; RR tr/tbCAS vs tfCAS:2.0, 95%CI(1.1-3.7); RR tr/tbCAS vs TCAR:3.0, 95%CI(1.5-6.2)] and hyperperfusion symptoms [tr/tbCAS:1.4%, tfCAS:0.8%, TCAR:0.2%; RR tr/tbCAS vs tfCAS:2.1, 95%CI(1.0-4.2); RR tr/tbCAS vs TCAR:6.8, 95%CI(3.0-15)]. However, tr/tbCAS was associated with lower risk of any access-related bleeding complication [tr/tbCAS:1.8%, tfCAS:3.3%, TCAR:2.9%; RR tr/tbCAS vs tfCAS:0.42, 95%CI(0.20-0.88); RR tr/tbCAS vs TCAR:0.28, 95%CI(0.13-0.58)]. There were no significant differences for the individual endpoints of stroke or MI (Table).
CONCLUSIONS: Compared with tfCAS or TCAR, tr/tbCAS was associated with higher risk of in-hospital stroke/death and death, despite lower risk of access-related bleeding complications. These findings warrant further investigation and call into question the expected benefits of a tr/tbCAS approach, particularly if patients are reasonable candidates for tfCAS or TCAR. 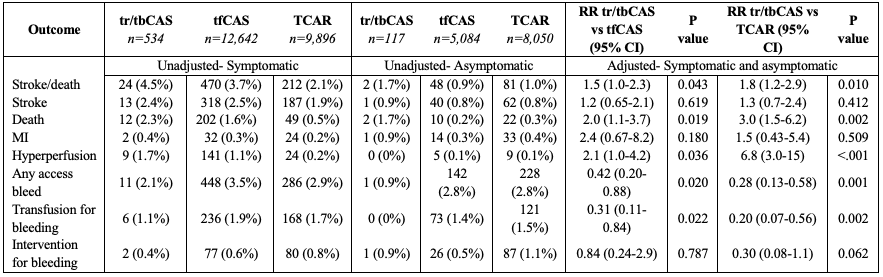
Back to 2022 Abstracts
