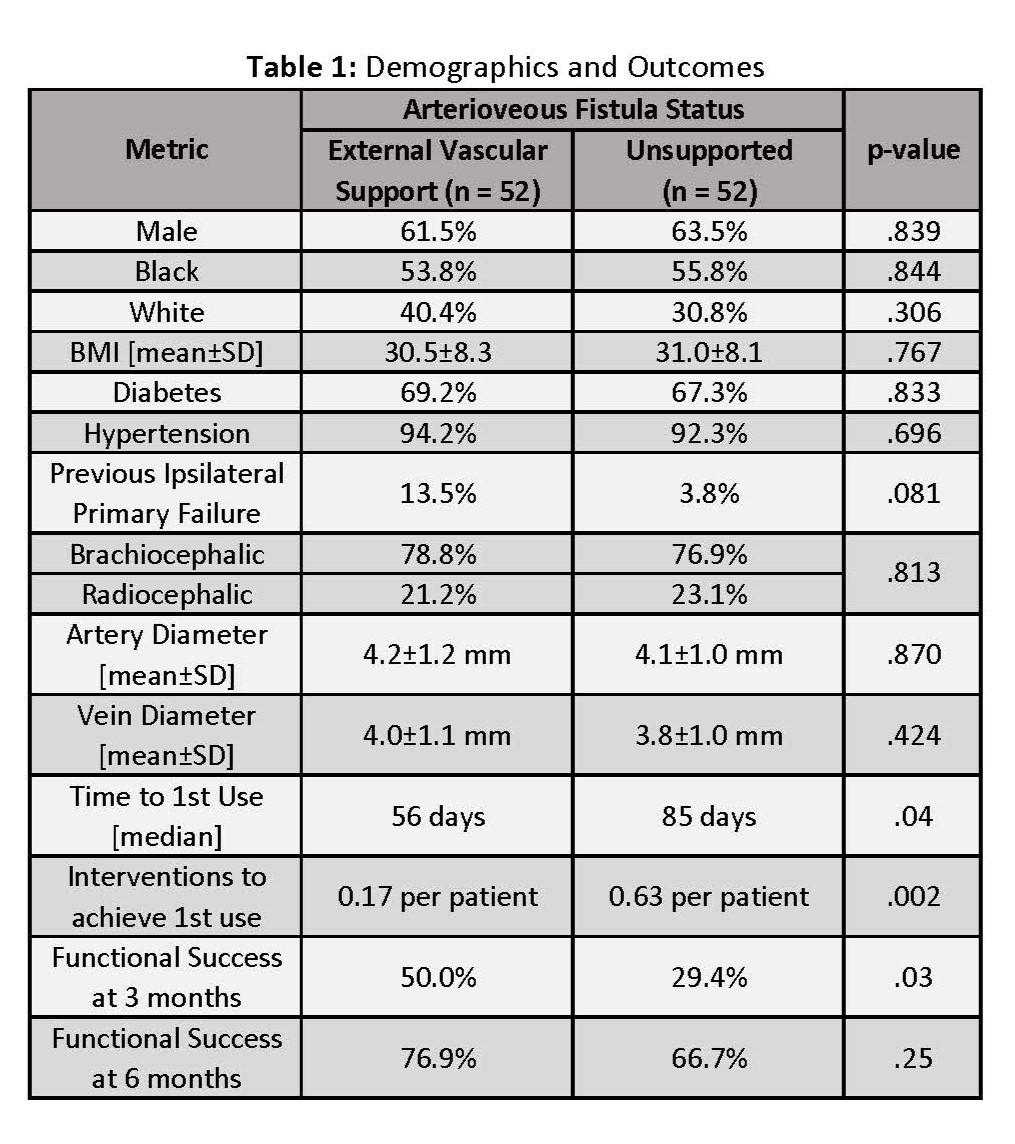
OBJECTIVES: This study aims to assess the functional outcomes of arteriovenous fistula (AVF) created with an external vascular support device as compared with rigorously matched controls of unsupported AVFs for End-Stage Renal Disease patients (ESRD). METHODS: The VasQ U.S. Pivotal Study (Laminate Medical, Tel Aviv) was a prospective, single-arm, multicenter study that enrolled patients from 2018 through 2019 to evaluate the safety and efficacy of creating AVFs with an external vascular support. Sites that enrolled more than six patients were invited to participate in a retrospective chart review of unsupported AVFs to compare to VasQ Study patients. Control patients were selected by sites utilizing the same inclusion/exclusion criteria as the VasQ Pivotal study, blinded to the individual patient outcomes, from a time period prior to study initiation. Comparison of baseline characteristics was performed to confirm population uniformity. Functional outcomes were evaluated of time and intervention rate prior to first use, as well as functional success defined as proportion of fistulas used for hemodialysis 3- and 6-months post AVF creation. RESULTS: Six VasQ U.S. Pivotal Study sites were able to participate, which provided 104 ESRD patients with 52 in the VasQ cohort and 52 in the unsupported cohort for the analysis. Both groups were well-matched with no significant difference in baseline parameters, including vessel diameters, as shown in Table 1. Analyses highlighted a significant reduction in median time (56 vs. 85 days, p = .04) with significantly fewer interventions to achieve first use (0.17 vs. 0.63, p = .002 per patient) in the VasQ cohort as compared to the unsupported cohort. Significantly more of the VasQ cohort achieved functional success by the 3-month timepoint (50% vs. 29.4%, p = .03) with a trend toward higher functional success rate at 6 months, albeit not statistically significant (76.9% vs. 66.7%, p = .25). CONCLUSIONS: The study offers encouraging evidence for the efficacy of external vascular support of AVFs, especially in lowering intervention rates and shortening the time to achieving functional success. If confirmed with future device experience, the improvement in these key functional outcomes will translate into clinically meaningful benefits for renal failure patients.