OBJECTIVES: This is a multi-center analysis of the safety and feasibility of the Unitary Stent Graft (USG) for endovascular debranched aortic repair (EDAR) of various thoracoabdominal aortopathies.
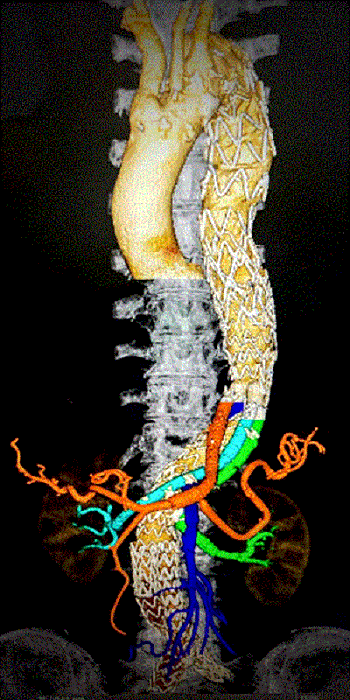
METHODS: Over 7 years, 139 consecutive patients at prohibitive risk for open surgery underwent EDAR for treatment of dissecting and non-dissecting thoracoabdominal aortic aneurysms (TAAA), including failed prior repairs. There were no anatomical screening failures. The USG is assembled using the Medtronic Endurant II platform and Gore Viabhan stents to separate flow proximal to zone 6 into a visceral limb and an infrarenal limb, thereby facilitating endoluminal bypasses to target mesenteric and renal vessels and infrarenal EVAR (FIGURE I). Two physician-sponsored investigational device exemption databases (G140207, G170036) and one pre-submission database (Q222702) were prospectively maintained and retrospectively reviewed for mortality and major adverse events (MAEs) at 30 days; technical success (defined as successful back-table assembly, delivery, and deployment of the USG; target vessel endoluminal bypasses; and infrarenal aortic interventions); and treatment success as assessed via clinical follow-up with imaging.
RESULTS: There were 2 (1.4%) short-neck (<10 mm) infrarenal aneurysms; 2 (1.4%) Type I, 9 (6.5%) Type II, 6 (4.3%) Type III, 99 (71.2%) Type IV, and 3 (2.2%) Type V Crawford TAAAs, and 18 (12.9%) chronic dissections. Overall, 7 (5%) EDARs were performed for failed prior repairs. Technical success was achieved in all (100%) including endoluminal bypasses to all 539 targeted vessels, with a 30-day primary and secondary patency rates of 98.7% and 99.8%, respectively. Thirty-day mortality was 3.6% (5 deaths). MAEs included paraplegia (n=1; 0.7%), stroke (n=6; 4.3%), bowel ischemia (n=4; 2.9%), renal failure (n=11; 9.2%), myocardial infarction (n=6; 4.3%), and respiratory failure (n=22; 15.8%). Median hospital stay was 7 days (interquartile range{IQR} 3.25 days). There were no type I or III endoleaks immediately or at the median follow-up of 360 days [{IQR 672 days}]. At median follow up, all-cause and aneurysm-related mortality were 23.0% (n=32) and 3.6% (n=5), respectively.
CONCLUSIONS: USG is a safe and effective, reproducible, off-the-shelf option for EDAR of various thoracoabdominal aortopathies, including failed prior repairs, in patients at prohibitive risk for open surgery and/or other endovascular therapies.