Objective To report results of a prospective, single-arm, registry-based study assessing the safety and performance of paclitaxel drug-coated balloon (DCB) for the treatment of superficial femoral artery (SFA) or popliteal artery ISR in a United States population.
Methods We conducted a prospective, non-randomized, multi-center, single-arm, post-market registry of the IN.PACT Admiral DCB for treatment of ISR lesions in the SFA/popliteal artery at 50 sites within the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) Registry from December 2016-January 2020. Clinical outcomes were assessed at 12, 24, and 36 months. The primary endpoint was target lesion revascularization (TLR) at 12-months. Secondary endpoints included technical success, target vessel revascularization (TVR), major limb amputation, and all-cause mortality. Results are presented as survival probabilities based on Kaplan-Meier survival estimates.
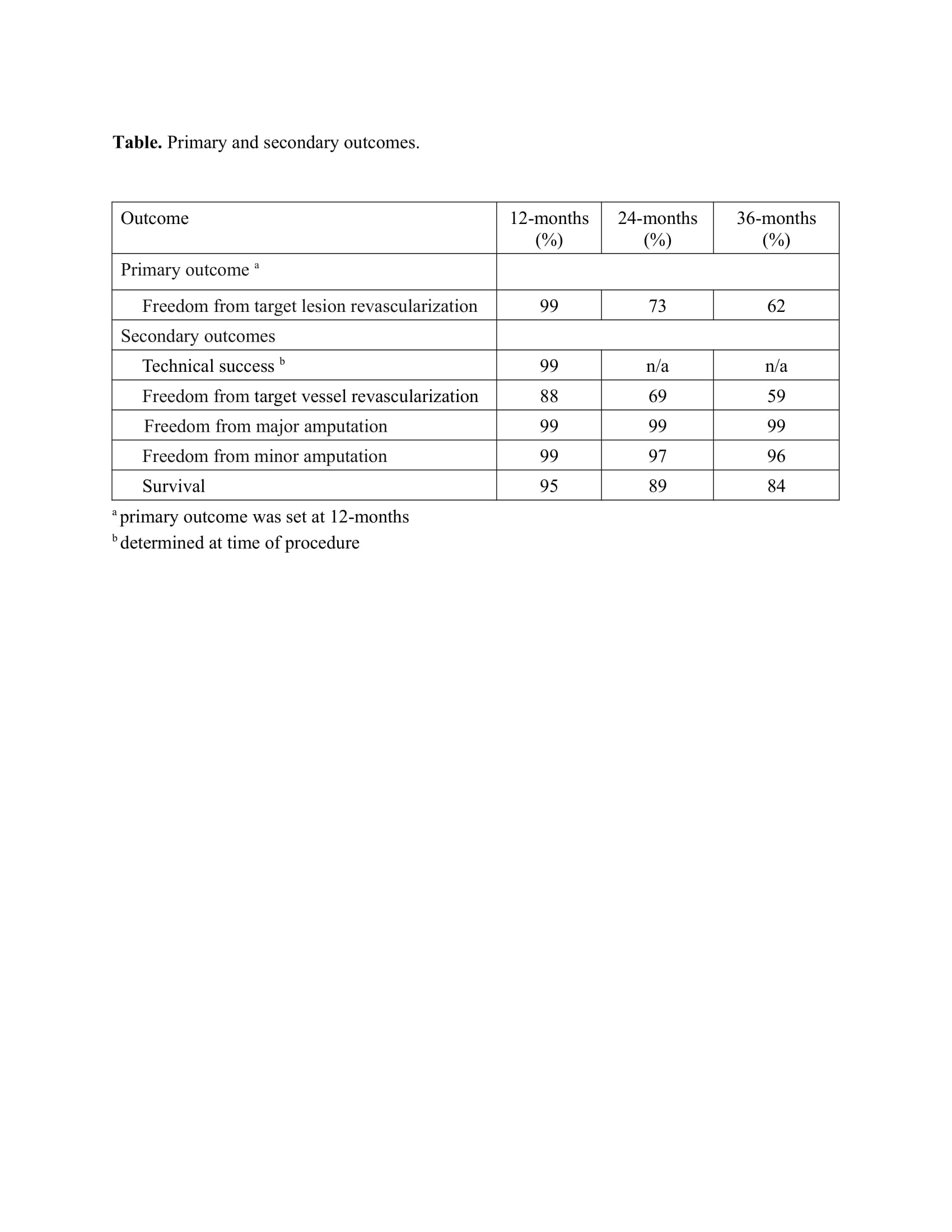
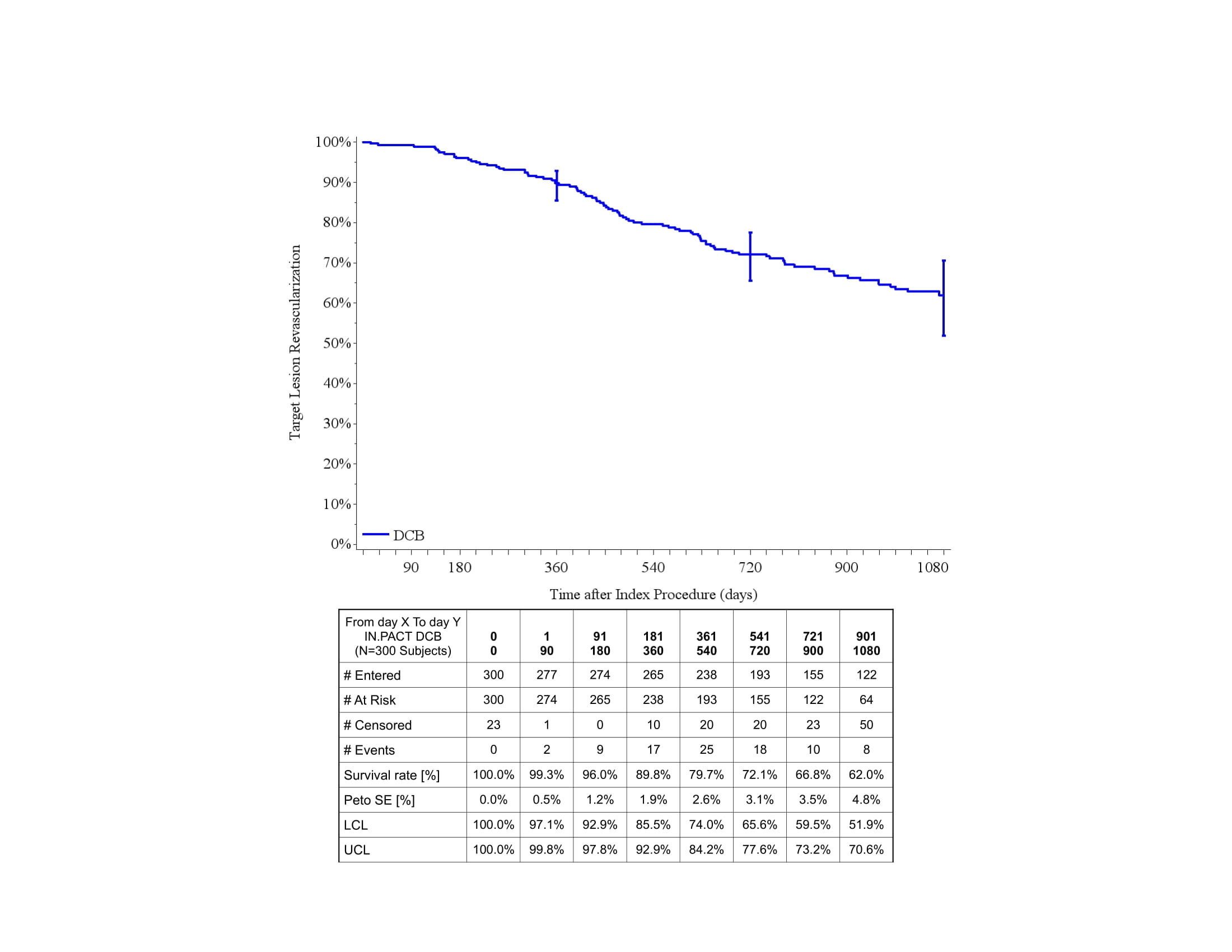
Results Patients (N=300) were 58% male with mean age of 68 ± 10 years. Diabetes was present in 56%, 80% presented with claudication and 20% with rest pain. Lesions included ISR of the SFA in 68%, SFA-popliteal in 26%, and popliteal arteries in 7%. The mean lesion length was 17.8 ± 11.8 cm. Occlusions were treated in 43% (mean occluded length 16 ± 10 cm). TASC type was 17% A, 29% B, 38% C, and 15% D. Technical success was 99% (Table). Re-stenting was used in 5% and thrombolysis in 0.6% of procedures. Based on Kaplan-Meier estimates freedom from TLR was 90%, 73% and 62% at 12-, 24- and 36 months (Figure). Freedom from TVR was 88%, 69% and
59%. Freedom from major target limb amputation was 99% at 12-, 24 and 36-months. Survival was 95%, 89% and 84% at 12-, 24- and 36 months.
Conclusion This post-market registry-based study of a paclitaxel DCB shows promising results in treating femoral-popliteal ISR with freedom from TLR of 90%, 73% and 62% at 12-, 24- and 36 months. These results demonstrate the ability of the SVS VQI to conduct post-market evaluation of peripheral devices in partnership with industry and federal regulators.