Back to ePosters
Multidisciplinary Care Includes Endovascular Abdominal Aortic Aneurysm Repair (EVAR) for Cancer Patients and Survivors in a Tertiary Referral Cancer Center
Tam T. Huynh, M.D.1, Steven Y. Huang, M.D.2, Reza Mehran, M.D.2, Kamran Ahrar, M.D.2.
1University of Texas MD Anderson Cancer Center and The Methodist Hospital, Houston, TX, USA, 2University of Texas MD Anderson Cancer Center, Houston, TX, USA.
OBJECTIVES: Advances in oncologic treatment and endovascular therapy have led to parallel improvement in patient outcome. However, the management of large abdominal aortic aneurysm (AAA) in cancer patients remains controversial. We conducted this study to determine the outcome of patients with AAA undergoing EVAR either prior to or after cancer treatment.
METHODS: Retrospective study of consecutive patients who underwent EVAR for non-ruptured aorto-iliac aneurysm > 5 cm in a tertiary referral cancer center between 09/2010 and 08/2013. Patient demographics, procedure-related complications, length of hospital stay, interval time to cancer treatment, and 30-day and intermediate-term outcome were reviewed.
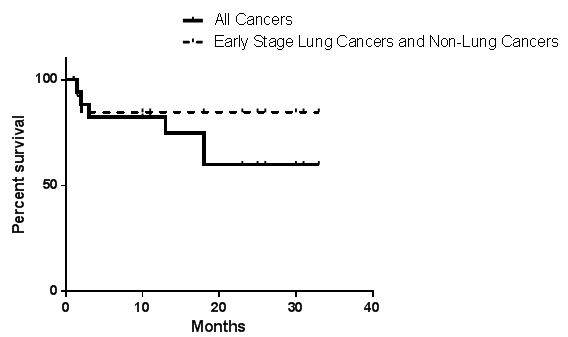
RESULTS: Twenty patients had EVAR and median follow-up was 15 months (range: 1- 33). All patients were male, either current or former tobacco smoker (mean 50 pack-years), with median age, 71.5 year-old (range 58-79). Fifty percent of the patients had hypertension, 40% COPD, and 35% coronary artery disease. Thirteen patients had active cancer, and 7 were in remission for at least 6 months (types of cancer: 10 bronchogenic, 6 lymphoma/melanoma, 2 gastric, 1 prostrate and 1 laryngeal). Median AAA size was 5.8 cm (range: 5.2-8.8). EVAR was successful in all patients. The initial 3 patients had femoral artery cut-down, and the remaining 17 had totally percutaneous access. The median length of hospital stay was 1 day (range: 1-5). All patients survived at 30-days post-EVAR. Access complications occur in 2/20 (femoral pseudoaneuryms). There were 6 endoleaks (type II); 4/6 resolved. The mean % of AAA diameter reduction was 19% at one- and 29% at 2-years. Mean interval time to begin or resume oncologic treatment was 26 days after EVAR (8-48). Six patients died while undergoing cancer treatment (4/6 had advanced lung cancer, 1/6 had sudden death. and 1/6 died of complications from gastrectomy). Fourteen patients are currently alive (figure).
CONCLUSIONS: Our study shows that EVAR is effective and safe in cancer survivors and patients undergoing active oncologic treatment. Prognosis was related to oncologic type and stage (worse in advanced lung cancer). Based on our results, we advocate that EVAR be part of the multidisciplinary strategic treatment plan for cancer patients with large AAA.
Back to ePosters

|


